A significant challenge in medical research is understanding why we fall ill. Why is it that some of us get certain diseases whilst others do not. Although we are genetically very different from each other (unless you count your identical twin), understanding the reasons behind variability in health does not just fall onto the obvious candidate – genetics; rather we have to consider another very important variable, the environment.
A report published in 2006 by the World Health Organisation states that 13 million deaths occur from environmental causes and up to 24% of these deaths are actually preventable [1]. A large number of these environmental factors are pollutants such as metals and hydrocarbons, whilst some exposures arise as a by-product of our agricultural efforts like the use of pesticides. To really understand how we fall ill from these environmental factors, we have to understand how we as biological entities respond to our environment.
We are equipped with a static toolkit – the genome
The environment is highly dynamic, from weather patterns, to the air that we breath and the availability of water. Whilst we are equipped with a static toolkit – the genome, we have to turn on and off certain genes depending on stresses imposed on us by the environment. A classical example is the heat shock response, which is involved in turning on genes and making proteins that help to protect your cells, from a variety of stresses from exposure to heavy metals, cytotoxic drugs and viral infections [2]. It’s pretty clever actually, it allows your cells to “brace themselves” through a tough time. However a really interesting point here, is how is this communicated?
This is where a nascent field of research is taking the limelight. Environmental epigenetics is being used to interrogate our relationship with the environment. What is epigenetics? It is the study of heritable changes in gene expression without changes in the DNA sequence. Through epigenetics, we are able to adjust the the expression of certain genes in response to an exogenous influence, i.e. the environment. This is achieved through a variety of mechanisms, broadly referred to as epigenetic modifications. Examples include adjusting the levels of methylation on DNA to silence genes and adding chemical groups to proteins called histones which act as a scaffold for your DNA. This allows the DNA to have varied accessibility to the molecular machines involved in expressing your genes and more importantly it allows for variation in the relative expression of certain genes at specific times in response environmental stimulus.
The traditional gene-environment model, is not enough.
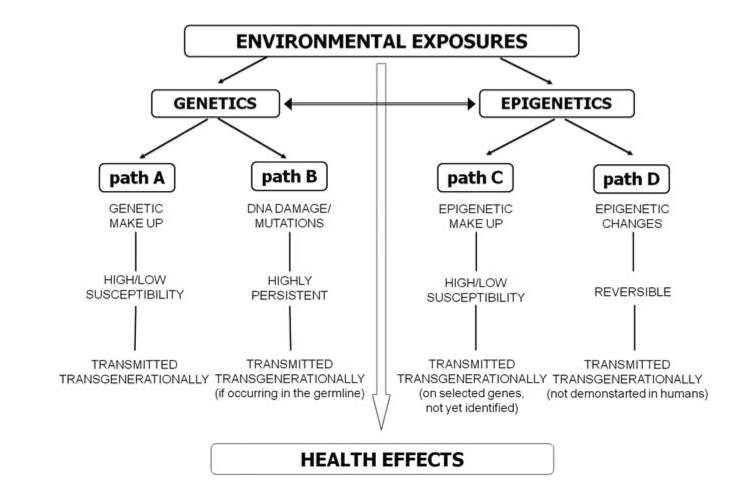
Traditionally, health outcomes were considered to occur from gene-environment interactions. In this model, diseases resulted from interactions between an individual’s genetic make up and the environmental factors. Those studying genetics have stood by the concept that the expression of a particular physical characteristic (phenotype) is variable and dependent on the environment to which the individual is exposed to. In this example, some people may have a relatively low risk in developing a disease in response to environmental factors, whilst others are more likely, purely due to their genetic differences or polymorphisms [*]. It has now become apparent this this approach is too simplified.
Recent research published in Nature’s Heredity, has stated that we should add an epigene-environment approach. In the epigene-environment framework, the relative differences in an individual imposed by epigenetic mechanisms are also important and of similar weight to our genetic differences. In this example, epigenetic differences, so the way in which I express my genes in response to the environment compared to you, may also adjust our susceptibility to diseases [3]. It also means that in addition to our genetic make-up, our epigenetic make-up will have an impact on our health in response to environmental exposures.
This approach is growing momentum due to the increasing evidence of epigenetic changes that occur as a result of environmental factors. Particulate matter and air pollution is believed to adjust the levels of DNA methylation of the iNOS gene, leading to negative health outcomes. Furthermore, environmental stresses during pregnancy can lead to permanent changes in epigenetic modifications, leading to stresses in the newborn child, which has recently been shown by studying women that were pregnant during the 9/11 attacks [4]. This idea of inheriting an experience with an associated health outcome is particularly alarming. In mice exposed to air from a steel manufacturing plant, it has been shown that the DNA in their sperm is hypermethylated and this persists even after removal of the exposure, suggesting that such epigenetic abnormalities can be transmitted transgenerationally [5]. In addition to DNA methylation, aberrant histone modification has also been identified as a result of exposure to metals such as, nickel, chromium, lead and arsenic – the latter is found in abundance in the water table of developing countries, allowing for a chronic exposure. As histone modifications can regulate the levels of gene expression, environmental factors that impinge on this process can be destructive often leading to cancer and neurodegenerative disorders [6].
Although the epigene-environment framework described above has yet to to be formalised, there is growing evidence that epigentics may assist us in predicting the risks and susceptibility of an individual to develop disease. The challenges now are to determine what the epigenetic alterations are and also to understand the physiological meaning of these events in the context of disease.
References:
1. Pruss-Ustun A, Corvalan C., (2006). Preventing disease through healthy environments. Towards an estimate of the environmental burden of disease. Geneva, World Health Organization (WHO).
2. Santoro, M., (2000). Heat shock factors and the control of the stress response. Biochemical Pharmacology, 59(1), pp.55–63.
3. Bollati, V. & Baccarelli, A., (2010). Environmental epigenetics. Heredity, 105(1), pp.105–112.
4. http://news.bbc.co.uk/1/hi/health/4508879.stm
5. Yauk C, Polyzos A, Rowan-Carroll A, Somers CM, Godschalk RW, Van Schooten FJ et al. (2008). Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc Natl Acad Sci USA 105: 605–610.
6. Fragou, D. et al., 2011. Epigenetic mechanisms in metal toxicity. Toxicology Mechanisms and Methods, 21(4), pp.343–352.
*(to know more about polymorphisms please refer to the Call of Duty article)
I have a cold. I’m pretty sure i caught it from my germ-infested siblings. Yet another way to annoy me. Damn them!
Sounds like you’re living in a hostile environment! The true question is this though, if you managed to catch something from one of your siblings, does that mean everyone that comes into contact with them will also fall ill? Some of us don’t, whilst some of us do. We all have different immune systems, when it comes to germs, but in the case of complex diseases like neurodegenerative disorders and exposure to toxicants, considering our epigenetic make-up is just as important as our genetic make-up. 🙂
Minimolecule, it’s great that your blog doesn’t shy away from the big questions. Whilst the question of why we fall ill is an admirable one, I would suggest that the more pertinent question is how do we prevent illness? It is often thought that a full and complete answer to the former is needed before we can move on to the latter (it is intuitive after all), but medical historians and many medical practitioners know better (counter intuitive research is the most interesting research of all).
The question of permanence and the quality of childhood can also lead to interesting counter intuitive findings. This is because what may seem a unpleasant experience might actually have beneficial effects subsequently. To take an immunological example, if Shimi’s already had chicken pox in childhood, she is equipped in adulthood for a chickenpox free life. To take a physiological example, if you train for a marathon by running hard repeatedly, you’d be prepared for race day. To take a cognitive example, revising hard sets you up for exams and so on. Some of the utility calculations and associated cost discounting might make John Stuart Mill proud, but I’m not sure that’s how we do it in real life. Figuring out when a difficult or unpleasant experience is worth it for future pay off is not something we do all that often. This is why the permanence of past experiences is a question science can address, but it will struggle to tell us much about their quality or when those experiences are worthy or justified. For me, the quality question requires the input of moral philosophers, irrespective of whether scientists admit that or not. I believe Amartya Sen came to the same conclusion.
Sorry for the late reply. The plasticity and permenance of epigenetic changes is an area of constant debate. However some chemical modifications of DNA, particularly DNA methylation has become synonymous with gene silencing. Essentially areas of the genome that are to be turned off are highly methylated (addition of a chemical group). This is particularly important in genetic dosage compensation in females that have two X chromosomes. Because they essentially have two of the same type of chromosome, as a result one chromosome is heavily methylated to ensure the genes that are expressed are not doubled up, and also not double the number of their male counterparts. Regarding your in-utero ideas, this is a very hot topic in epigenetics. In fact, some of the earliest discoveries of epigenetic phenomena originated from charting the progress of child development from aberrant in-utero environmental factors. A classic example is the 1944-45 World War 2 food embargo in Holland. This lead of a number of pregnant mothers having poor nutrition. It then later transpired that this environment, lead to poor DNA methylation patterns in the developing embryo and consequently these children had a number of negative health outcomes, even though their upbringing was not within the same environmental context as their parents (i.e the food embargo was lifted). Our understanding of these forms of inheritance patterns are still nascent and it is an area of intense research. If you would like to know more about the study, I recommend listening to this BBC audio-documentary about epigenetics (link below).
http://www.bbc.co.uk/programmes/b00wdjgl
I think your site is pretty good, keep up the good work and I will come back for more.
I have a cold. I’m pretty sure i caught it from my germ-infested siblings. Yet another way to annoy me. Damn them!
Haha! Sorry to hear that! Hope you feel better soon 🙂